 https://globalcompliancepaneltraining.files.wordpress.com/2018/02/osha-1.jpg?w=150 150w,…
https://globalcompliancepaneltraining.files.wordpress.com/2018/02/osha-1.jpg?w=150 150w,…
The Cutting Edge of Medical Technology Content, Community & Collaboration
All Blog Posts Tagged 'to' (24)
 How to Reduce Stress in the Work Place
How to Reduce Stress in the Work Place
Workplace health and well being is a rising public health concern. Poor employee health can be costly to the organization (e.g. absenteeism, poor productivity, reduced attention and workplace safety).
Chronic stress has been shown to impinge decision-making, reduce concentration, immune system responses, and job satisfaction, cause psychological distress (e.g. depression and anxiety) and coronary heart disease, and negatively impact client rapport and personal…
ContinueAdded by Roger Steven on November 21, 2018 at 7:18am — No Comments
 Concerned about health and safety on the job?
Concerned about health and safety on the job?
Added by John Robinson on February 20, 2018 at 4:55am — No Comments
 What is Emergency Planning and Community Right-to-Know Act (EPCRA)?
What is Emergency Planning and Community Right-to-Know Act (EPCRA)?
The Emergency Planning and Community Right-to-Know Act is a significant milestone in the detection and prevention of hazardous chemicals. The US Congress enacted this law in the wake of the Bhopal disaster in India. It seeks to involve…
Added by John Robinson on February 8, 2018 at 5:03am — No Comments
 What is an Environmental Impact Statement?
What is an Environmental Impact Statement?
 https://globalcompliancepaneltraining.files.wordpress.com/2018/02/environmental-impact-statement.jpg?w=860&h=752 860w,…
https://globalcompliancepaneltraining.files.wordpress.com/2018/02/environmental-impact-statement.jpg?w=860&h=752 860w,…
Added by John Robinson on February 6, 2018 at 5:03am — No Comments
 What is Accounting Fraud and How to Prevent Financial Statement Fraud
What is Accounting Fraud and How to Prevent Financial Statement Fraud

Key Takeaway: An understanding of what is accounting fraud and how to prevent financial statement fraud is an…
Added by John Robinson on February 5, 2018 at 4:54am — No Comments
 Health Education England launches online workshop on improving digital readiness
Health Education England launches online workshop on improving digital readiness

Health Education England is launching an online workshop to gather views on digital readiness.
The organisation is working in collaboration with Digital Health and innovation and crowdsourcing agency Clever Together on the online workshop, which forms part of the…
Added by John Robinson on November 14, 2017 at 5:32am — No Comments
 Murj wants to give data collection from implantable medical devices an upgrade
Murj wants to give data collection from implantable medical devices an upgrade

Murj, a new company backed by $4.5 million in new venture financing, is looking to make data collection from implantable heart monitoring and management devices easier and more manageable.
The company was founded by a former Medtronic sales rep who’d previously worked as a product manager on Apple’s iPads. After a few years in sales, Murj founder Todd…
Added by John Robinson on November 8, 2017 at 5:39am — No Comments
 Testing GxP system that is FDA-compliant
Testing GxP system that is FDA-compliant

Process validation is the method of using data from stages ranging from the process design stage to production, to ensure that the process that is being used can deliver consistently high-quality products. Companies that come under the life sciences must comply with GxP regulations to ensure that their processes meet the regulatory guidelines to be GxP compliant.…
Added by John Robinson on November 8, 2017 at 5:37am — No Comments
 Process Development and Validation rest on the right Design of Experiments and Statistical Process Control
Process Development and Validation rest on the right Design of Experiments and Statistical Process Control

The application of DOE and SPC to the development, design and monitoring of manufacturing and testing requires the use of procedures. Why? It is because in a recent guidance document on Process Validation, the FDA has named the Quality Unit as being responsible in the review and interpretation of DOE and SPC studies.
The Quality…
Added by John Robinson on November 2, 2017 at 6:49am — No Comments
 HR Tech: President of First U.S. Firm to Microchip Employees Will Speak in Dallas on Workplace Wearables
HR Tech: President of First U.S. Firm to Microchip Employees Will Speak in Dallas on Workplace Wearables
[Illustration: Aelitta/istockphoto]
What if your employer asked you to implant a microchip under your skin to help them keep track of you?
Three Square Market…
Added by John Robinson on October 4, 2017 at 6:41am — No Comments
 OSHA Proposes Extending Compliance Deadline for Crane Operator Certification Requirements to 2018
OSHA Proposes Extending Compliance Deadline for Crane Operator Certification Requirements to 2018

The Occupational Safety and Health Administration (OSHA) today issued a Notice of Proposed Rulemaking to extend the employer’s responsibility to ensure crane operator competency and enforcement for crane operator certification to Nov. 10, 2018.
OSHA…
Added by John Robinson on September 5, 2017 at 7:29am — No Comments
 How to create processes and procedures to implement them?
How to create processes and procedures to implement them?
Product Risk Management is a critical aspect of ensuring medical devices are safe and effective for intended uses. This course will help you understand the regulatory requirements, including ISO14971.
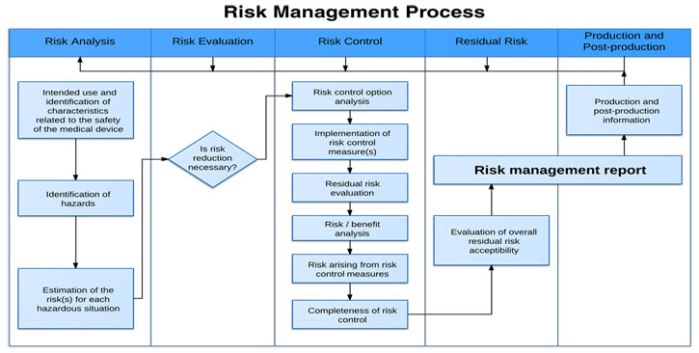
You’ll learn techniques that can help you identify hazards and potential harms. You’ll learn how to mitigate risk and effectively monitor risk to ensure your customers receive safe and effective products. A rigorous…
ContinueAdded by Adam Fleming on July 19, 2017 at 5:47am — No Comments
 How to control those risks and monitor the effectiveness of the controls put in place
How to control those risks and monitor the effectiveness of the controls put in place
Overview of this session Product Risk Management is a critical aspect of ensuring medical devices are safe and effective for intended uses. This course will help you understand the regulatory requirements, including ISO14971, and how to create processes and procedures to implement them.
You’ll learn techniques that can help you identify hazards and potential harms. You’ll learn how to mitigate risk and effectively monitor risk to ensure your customers receive safe and effective…
ContinueAdded by Adam Fleming on July 13, 2017 at 5:43am — No Comments
 Writing and implementing effective SOP’s
Writing and implementing effective SOP’s
Added by John Robinson on June 6, 2017 at 6:36am — No Comments
 EPA’s new Revised Section 608 Refrigerant Management Regulations
EPA’s new Revised Section 608 Refrigerant Management Regulations
In late 2016, the US Environmental Protection Agency (EPA) revised Section 608, which deals with refrigerant management regulations. A number of requirements under this section are set to change. They concern the handling requirements that are applicable to ozone depleting refrigerants, and fortify the existing regulations by…
Added by Adam Fleming on May 16, 2017 at 6:33am — No Comments
 All about human error and ways of reducing it
All about human error and ways of reducing it
There is the saying that after all is said and done, to err is human. In a philosophical sense, it can have numerous interpretations, but in the field of Good Manufacturing Practice, it cannot be taken with a pinch of fatalism. GMP requires everything to be precise and scientific to a T. GMP is not an area that leaves anything to human error. Being an area of science, GMP has no place for imagination and chance.…
Added by Adam Fleming on May 8, 2017 at 7:48am — No Comments
 The art of writing effective audit observations
The art of writing effective audit observations
Audits are a means of evaluating operations and other functions of an organization. ISO 19011:2011 Guidelines for Auditing Management Systems describes audits as a process used for gathering the evidence of verifiable documents and map their suitability, alignment and fulfilment with the company’s policies and procedures.
An audit is an important tool that helps organizations to analyze opportunities, implement best practices, and assess all the important factors in…
Added by Adam Fleming on April 27, 2017 at 6:29am — No Comments
 Tips and Suggestions on interacting with FDA Officials and Premarket Approval (PMA)
Tips and Suggestions on interacting with FDA Officials and Premarket Approval (PMA)
Added by John Robinson on April 25, 2017 at 6:18am — No Comments
 Establishing Latest Quality Systems in medical device and pharmaceutical industries
Establishing Latest Quality Systems in medical device and pharmaceutical industries
Establishing Quality Systems is one of the central aspects of a medical device and/or pharmaceutical organization. Establishment of Quality Systems is also a regulatory requirement, as set out by the FDA and the ISO.
The process of establishment of Quality Systems for FDA-regulated medical devices industries is set out in 21 CFR Part 820. Further, the ISO has its standard for how to establish Quality Systems in medical devices industries –the ISO 13485 standard…
Added by Adam Fleming on March 28, 2017 at 9:06am — No Comments
 The importance of Design of Experiments (DoE)
The importance of Design of Experiments (DoE)
Design of Experiments (DoE) is an important component in many industries. It is a series of tests or runs that is carried out repeatedly and consistently over a period of time, and its outputs or responses, observed. Design of Experiments is very important in industry to help arrive at an understanding of the predictability and reproducibility of an experiment.
Design of…
Added by Adam Fleming on March 8, 2017 at 5:12am — No Comments
Latest Blog Posts
- How to Reduce Stress in the Work Place
- Concerned about health and safety on the job?
- What is Emergency Planning and Community Right-to-Know Act (EPCRA)?
- What is an Environmental Impact Statement?
- What is Accounting Fraud and How to Prevent Financial Statement Fraud
- Health Education England launches online workshop on improving digital readiness
- Murj wants to give data collection from implantable medical devices an upgrade
Most Popular Blog Posts
- What is Accounting Fraud and How to Prevent Financial Statement Fraud
- Murj wants to give data collection from implantable medical devices an upgrade
- Tips and Suggestions on interacting with FDA Officials and Premarket Approval (PMA)
- How to Reduce Stress in the Work Place
- Concerned about health and safety on the job?
- What is an Environmental Impact Statement?
- What is Emergency Planning and Community Right-to-Know Act (EPCRA)?
Monthly Archives
2022
2020
2019
2018
- December (5)
- November (8)
- October (8)
- September (7)
- August (8)
- July (9)
- June (3)
- April (7)
- March (7)
- February (21)
- January (32)
2017
- December (11)
- November (39)
- October (64)
- September (60)
- August (34)
- July (30)
- June (22)
- May (40)
- April (16)
- March (12)
- February (18)
- January (15)
2016
2015
2014
2013
2012
- October (3)
- September (1)
- August (9)
- July (8)
- June (8)
- May (11)
- April (6)
- March (11)
- February (1)
- January (6)
2011
- December (2)
- November (3)
- October (7)
- September (3)
- July (4)
- June (4)
- April (7)
- March (4)
- February (3)
- January (7)
2010
- December (8)
- November (11)
- October (6)
- September (11)
- August (18)
- July (28)
- June (24)
- May (10)
- April (31)
- March (44)
- February (45)
- January (85)
2009
- December (104)
- November (63)
- October (44)
- September (68)
- August (53)
- July (37)
- June (50)
- May (43)
- April (63)
- March (106)
- February (36)
- January (15)
2008
1999
- November (5)
© 2024 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()

