The Cutting Edge of Medical Technology Content, Community & Collaboration
Key Takeaway:
GMPs are critical for the manufacture and distribution of foods, drugs and other pharmaceutical products. These need to be implemented diligently by organizations that are involved in these products.
Quality is a great concern for a manufacturer and consumer of any product. To ensure that quality is maintained across a variety of products ranging from foods to pharmaceutical products; a few guidelines are required. These guidelines are collectively termed Good Manufacturing Practices(GMP).
Not a uniform set of standards
It needs to be understood that GMPs are not a uniform or homogeneous set of rules for everyone to follow. These are general principles laid out for ensuring that there is a minimum level of quality requirements to be fulfilled.
Agencies that control authorization and licensing for manufacture and sale of food, drug products, and active pharmaceutical products recommend these guidelines. These guidelines can be accomplished in many ways, and it is up to the organization to find out the one that suits it best and implement that system.
In essence, Good Manufacturing Practices can mean the following:
- GMPs are set practices that manufacturers need to put in place to ensure that their products meet specified quality standards.
- GMP guidelines consist of the minimum requirements that food product, drug or pharmaceutical manufacturers have to meet to assure that their products are of the prescribed quality and cause no harm or risk to those who consume them or the public at large
- Regulatory agencies in several countries oversee their respective countries’ and global Good Manufacturing Practices. Good Laboratory Practices (GLP) and Good Clinical Practices (GCP) are usually analogous to GMP
- In many countries, legislations require pharmaceutical and medical device manufacturers to comply with GMP procedures. Many require these organizations to create their own GMP guidelines that are line with their legislations.
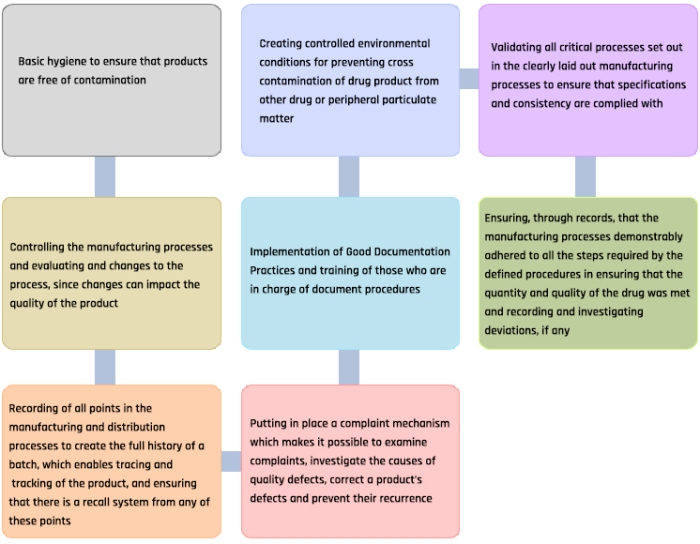
Basic points in GMP guidelines
Views: 19
Comment
© 2026 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()
You need to be a member of MedTech I.Q. to add comments!
Join MedTech I.Q.