The Cutting Edge of Medical Technology Content, Community & Collaboration
How to apply statistics to manage risks and verify/validate processes in R&D, QA/QC, and Manufacturing, with examples derived mainly from the medical device design/manufacturing industry. The flow of topics over the 2 days is as follows:
- ISO standards and FDA/MDD regulations regarding the use of statistics.
- Basic vocabulary and concepts, including distributions such as binomial, hypergeometric, and Normal, and transformations into Normality.
- Statistical Process Control
- Statistical methods for Design Verification
- Statistical methods for Product/Process Qualification
- Metrology: QC Sampling Plans the statistical analysis of measurement uncertainty, and how it is used to establish QC specifications
- How to craft “statistically valid conclusion statements” (e.g., for reports)
- Summary recommendations
The various statistical methods used to support such activities can be intimidating. If used incorrectly or inappropriately, statistical methods can result in new products being launched that should have been kept in R&D; or, conversely, new products not being launched that, if analyzed correctly, would have met all requirements. In QC, mistakenly chosen sample sizes and inappropriate statistical methods may result in purchased product being rejected that should have passed, and vice-versa.
This provides a practical approach to understanding how to interpret and use more than just a standard tool-box of statistical methods; topics include: Confidence intervals, t-tests, Normal K-tables, Normality tests, Confidence/reliability calculations, Reliability plotting (for extremely non-normal data), AQL sampling plans, Metrology (i.e., statistical analysis of measurement uncertainty ), and Statistical Process Control. Without a clear understanding and correct implementation of such methods, a company risks not only significantly increasing its complaint rates, scrap rates, and time-to-market, but also risks significantly reducing its product and service quality, its customer satisfaction levels, and its profit margins.
- FDA, ISO 9001/13485, and MDD requirements related to statistical methods
- How to apply statistical methods to manage product-related risks to patient, doctor, and the designing/manufacturing company
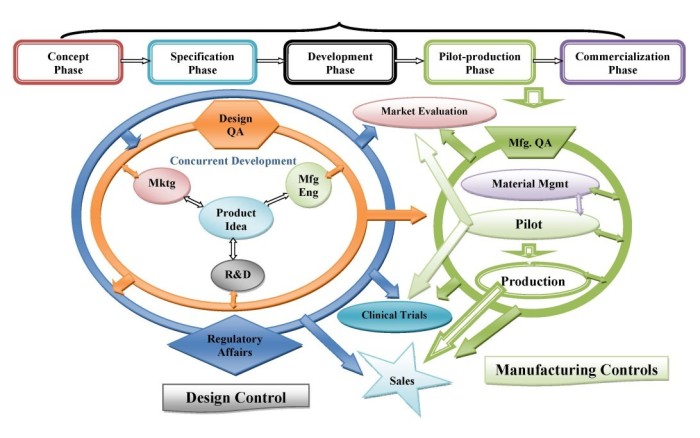
- Design Control processes (verification, validation, risk management, design input)
- QA/QC processes (sampling plans, monitoring of validated processes, setting of QC specifications, evaluation of measurement equipment)
- Manufacturing processes (process validation, equipment qualification)
 https://globalcompliancepaneltraining.files.wordpress.com/2019/01/product-development-process-e1395175968927.jpg?w=150 150w, https://globalcompliancepaneltraining.files.wordpress.com/2019/01/p... 300w, https://globalcompliancepaneltraining.files.wordpress.com/2019/01/p... 768w, https://globalcompliancepaneltraining.files.wordpress.com/2019/01/p... 1024w, https://globalcompliancepaneltraining.files.wordpress.com/2019/01/p... 1092w" sizes="(max-width: 700px) 100vw, 700px" />
https://globalcompliancepaneltraining.files.wordpress.com/2019/01/product-development-process-e1395175968927.jpg?w=150 150w, https://globalcompliancepaneltraining.files.wordpress.com/2019/01/p... 300w, https://globalcompliancepaneltraining.files.wordpress.com/2019/01/p... 768w, https://globalcompliancepaneltraining.files.wordpress.com/2019/01/p... 1024w, https://globalcompliancepaneltraining.files.wordpress.com/2019/01/p... 1092w" sizes="(max-width: 700px) 100vw, 700px" />
- QA/QC Supervisor
- Process Engineer
- Manufacturing Engineer
- QC/QC Technician
- Manufacturing Technician
- R&D Engineer
Views: 29
Comment
© 2026 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()
You need to be a member of MedTech I.Q. to add comments!
Join MedTech I.Q.