The Cutting Edge of Medical Technology Content, Community & Collaboration
A phase 3 trial studying the effects of tolvaptan has found that the drug slowed the rate of decline in kidney function in patients with polycystic kidney disease…
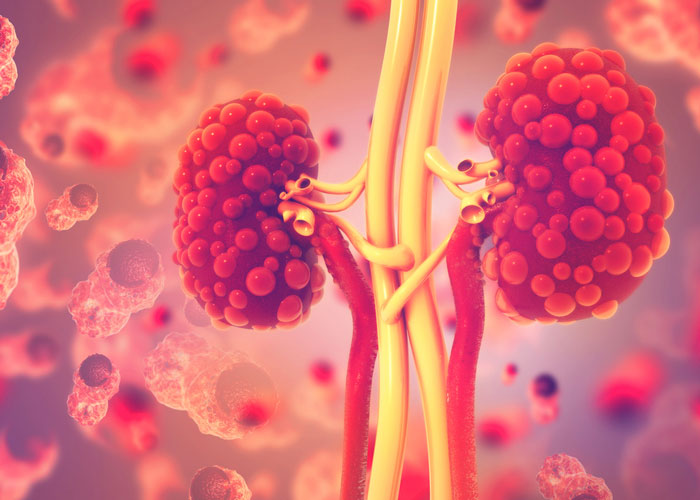
A phase 3 trial studying the effects of tolvaptan has found that the drug slowed the rate of decline in kidney function in patients with the most common form of polycystic kidney disease, a condition with no cure.
This is the first treatment that targets a mechanism that directly contributes to the development and growth of the kidney cysts in autosomal dominant polycystic kidney disease
The results of the trial demonstrated tolvaptan’s ability to intervene in a way that slows kidney function decline in this population.
“This is the first treatment that targets a mechanism that directly contributes to the development and growth of the kidney cysts in autosomal dominant polycystic kidney disease,” says Dr Vicente Torres, director of Mayo Clinic’s Translational Polycystic Kidney Disease Center. “This in effect means it may delay the need for a kidney transplant or dialysis in patients with this disease.”
Autosomal dominant polycystic kidney disease is an inherited condition that affects 1 in every 500 to 1,000 individuals in the U.S. This disease is found in all races and sexes. Autosomal dominant polycystic kidney disease, which is the fourth most common cause of end-stage kidney disease, requires dialysis or a kidney transplant.
The disease causes a slow but relentless growth of cysts that damage the kidneys. In addition to negatively affecting the quality of life, the condition also causes hypertension and painful complications. The cysts, which can damage kidneys with their size, can develop in other organs, especially the liver.
Approximately half of individuals with autosomal dominant polycystic kidney disease eventually will require dialysis or kidney transplant by age 60.
Views: 9
Comment
© 2024 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()
You need to be a member of MedTech I.Q. to add comments!
Join MedTech I.Q.