The Cutting Edge of Medical Technology Content, Community & Collaboration
Lyophilization is the clinical term for freeze-drying. In the simplest terms, it can be described as a technical process in which the moisture level of many materials is reduced, most times frozen, for the purpose of creating a new product or an ingredient.
When an injectable drug is freeze-dried -this is the process of lyophilization -its compounds are stabilized. This reconstitution process is helpful and necessary because substances in the drug get affected by outside or natural factors such as sunlight, oxygen, and pH values. These influences can bring down the effectiveness of these materials and molecules. It is to help them weather these countering factors that lyophilization is used. The aim of lyophilization is to make the best use of these compounds by preserving and protecting them in the form that can be used on patients.
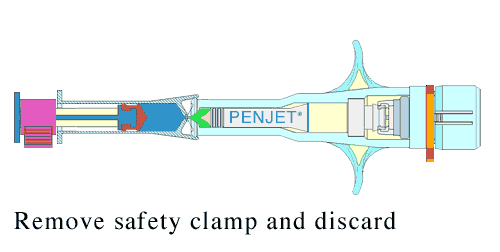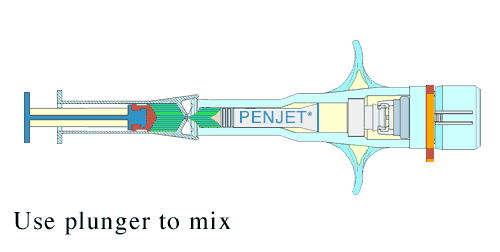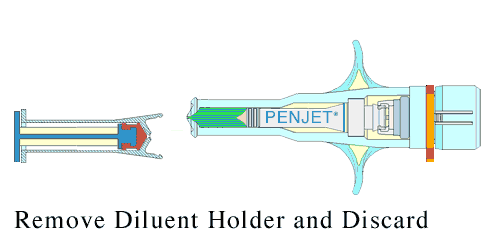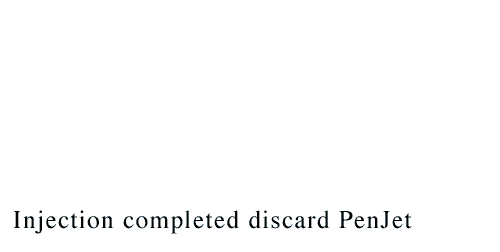
Lyophilization not only shields the product from unwanted biological activity; it also extends shelf life, and can bring about greater precision in the dosage. This reconstitution is done just before administration.
Where is lyophilization technology applied?
Lyophilization technology is used in a number of applications. The most prominent ones that use lyophilization include biotech drugs, as well as a few complex or very sensitive molecules.
Many industries are in a state of flux, in that new technological keep coming up every now and then. Lyophilization should keep abreast of these technologies and help businesses in these industries to innovate their products to meet the market needs.
Is lyophilization a new process?
No. It may surprise many that its roots can be located to more than a millennium, to ninth-century Asia! However, it has regained some prominence of late, with the rise in the use of compounds, chemicals, molecules and other related substances in our daily lives. Today, nearly a third of products in the parenteral segment that come to the FDA for approvals are for lyophilized drugs. Market watchers speculate that in the very near future; this proportion to set to go up to 50 percent.
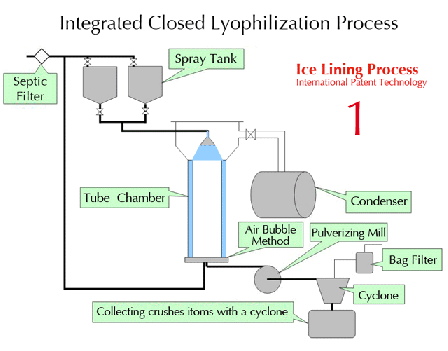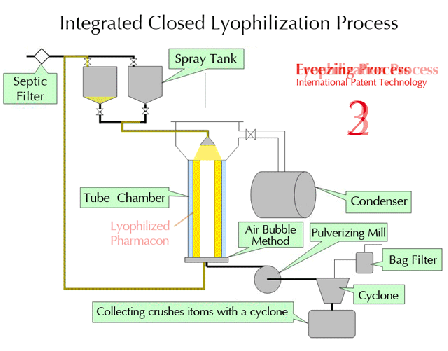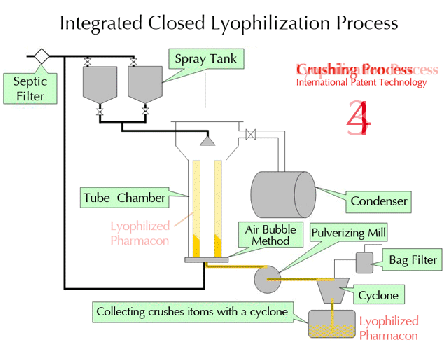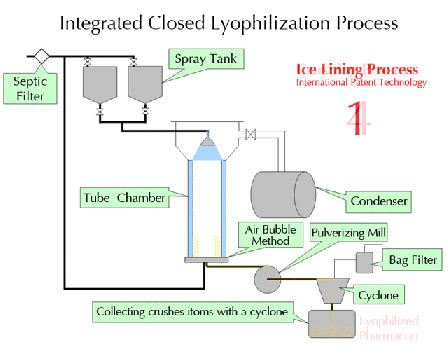
Characteristics of lyophilization
- Products that are lyophilized can be expected to maintain their stability for 2 to 3 years in ideal conditions
- Lyophilization calls for a high degree of expertise on the part of the manufacturer, since these drugs are derived out of formulations that are very complex
- Labs form lyophilization cycles that help in optimizing its efficacy. They seek a reduction in the residual moisture, which is something that has to be done for every product. This process usually begins at a product’s clinical development stage and gets finessed at the time of commercial manufacture.
Views: 46
Comment
© 2025 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()
You need to be a member of MedTech I.Q. to add comments!
Join MedTech I.Q.