The Cutting Edge of Medical Technology Content, Community & Collaboration
Nitinol-based Medical Devices 2017 Global Market Expected to Grow at CAGR 10.50 % and Forecast to 2021
About Nitinol-based Medical Devices
Nitinol-based medical devices are made of nitinol, an alloy of nickel and titanium. The use of nitinol-based stents and guidewires has improved procedural outcome. The nitinol alloys are corrosion resistant and are highly accepted in the medical device industry. The global medical devices market has been growing extensively over the years. With increasing investment in research and development (R&D) for manufacturing…
ContinueAdded by Adam Fleming on September 19, 2017 at 6:05am — No Comments
A common technique plots the data to help detect trends, cycles, and shifts
Some of the functions of medical device manufacturers include:
o Analyzing complaints
o Processing data
o Evaluating nonconformances
o Utilizing other quality data sources.
The main purpose of this analysis, done using appropriate statistical methodology, under §820.100, is to identify the cause of nonconforming products and other quality problems. Time series analysis is one such family of these tools. Also called trending analysis,…
Added by Adam Fleming on September 18, 2017 at 6:49am — No Comments
A clear process for compliant laboratory OOS investigations
The core of successful operation by a drug maker is laboratory testing. current Good Manufacturing Practices (cGMP) regulations require a drug manufacturer to use laboratory testing as a tool to validate that everything that goes into a laboratory product, such as in-process materials, finished materials, and containers adhere to set specifications. When all these are done, a major challenge for laboratories is in how to deal with a test that shows an…
Added by Adam Fleming on September 12, 2017 at 5:59am — No Comments
How to Recognize the Hazards of Blood Borne Pathogens
Bloodborne pathogens are those microorganisms present in the human blood that carry infection. These infections can cause disease in humans. The major bloodborne pathogens that cause infections in humans are:
o Hepatitis B (HBV)
o Hepatitis C (HCV)
o Human immunodeficiency virus (HIV)
Although these are the main disease causing pathogens; there are many more. So, hospital staffs who deal with patients who are infected by…
Added by Adam Fleming on September 8, 2017 at 5:46am — No Comments
What is the legal language of the FDA form 1572 or Device equivalent?
Form FDA 1572 is one of the primary documents needed when carrying out a clinical trial. Also called the Statement of Investigator; Form FDA 1572, called just 1572 informally, is a contract between the Principal Investigator (PI) and the FDA. This form contains all details of the subjects, as well as commitments from the PI.
It is a contract in which the Principal Investigator, the person who is…
ContinueAdded by Adam Fleming on September 6, 2017 at 6:11am — No Comments
How to Achieve the best Outcome in an Audit
An internal audit, as we all know, is carried out for a number of specific purposes, the main one among which is to assess the adherence to the industry guidelines for quality and processes. Helping the organization meet the requirements of processes and standards, which are usually issued by regulatory agencies and other relevant bodies and boards, is the main aim…
ContinueAdded by Adam Fleming on September 4, 2017 at 5:36am — No Comments
cGMP Data Integrity is of Critical Importance
Of late, the FDA has been turning on the heat on manufacturers in the FDA-regulated industries that violate its regulations. It has a penchant for going after manufacturing facilities that show laxity in implementing current Good Manufacturing Practices (cGMP). This ardor is understandable. cGMP violations affect the quality of the product; hence the strictness, considering that it is patients who consume these products.

There are ways of ensuring that the product…
ContinueAdded by Adam Fleming on September 1, 2017 at 6:05am — No Comments
Techniques to Follow Which Increase the Confidence in the Audit
Auditing is a very important aspect of quality in all the areas of the medical sciences. An audit is the most reliable and foolproof method for evaluating the extent to which the various activities in an organization comply with the requirements and expectations the principal has from those to whom various processes are outsourced. Audits are the main tools for a quality unit to monitor and ensure that those activities are performed in compliance with the manufacturer commitments for quality…
ContinueAdded by Adam Fleming on August 29, 2017 at 5:27am — No Comments
Tool for Checking Corporate Revenue Accounts Frauds
Corporate revenue account fraud and account manipulation are serious issues for businesses in many parts of the world today. Although legislations such as Sarbanes Oxley have been passed with the intention of making corporates more accountable; corporate accounting is still vulnerable to manipulation and fraud. A company’s financial statement is a primary area in which a fraud can happen.
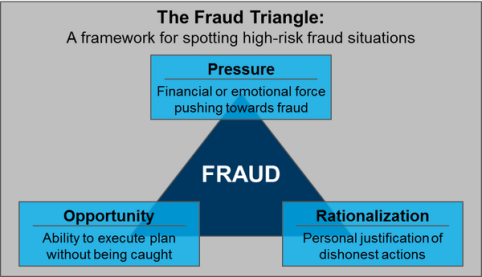
A financial statement is the elementary piece of documentation which reflects a…
ContinueAdded by Adam Fleming on August 22, 2017 at 5:28am — No Comments
The GDPR differs Significantly from EC Data Protection Directive 95/ 46
The General Data Protection Regulation (GDPR), which has been codified as Regulation (EU) 2016/679, is a very powerful law regarding the protection of data of the half billion people who live in the European Union (EU). Having come into effect as a result of the European Commission having adapted the proposal for its creation on January 25, 2012; it will replace Directive…
ContinueAdded by Adam Fleming on August 16, 2017 at 5:32am — No Comments
Relationship Between an EM Excursion Program and CAPA
The FDA’s regulations on cleanroom environmental monitoring (EM) are based on the assumption that any person who enters a cleanroom is likely and almost certain to have brought contaminants in one or another form with him. The FDA’s regulations on cleanroom environmental monitoring are built on the thinking that microorganisms can assail even the cleanest of systems, which is why its regulations on cleanroom…
ContinueAdded by Adam Fleming on August 9, 2017 at 5:39am — No Comments
Actions for Noncompliance of cGMPs in the Quality Control Laboratory
Quality controls in laboratories are a major area for which the FDA issues 483’s. A laboratory is the venue for many activities, all of them of varying importance to the product. When controls in laboratories are not up to the standard, such a laboratory could produce products that do not meet quality and processes expectations, and hence invite 483’s.

Issues with drug quality, drug integrity and data integrity, as well as data fabrication and human errors…
ContinueAdded by Adam Fleming on August 8, 2017 at 5:36am — No Comments
Latest Trends in Human Error Issues in the Industry
To say that the manufacturing industry is huge is to make a huge understatement. It is an activity that spans entire industries and is the lifeblood of many economies, right from advanced to developing ones. Despite the advancement in automation in the processes of many industries, manufacturing is still heavily dependent on human labor.
This makes manufacturing an activity that is prone to human error, because wherever there is human involvement, there is scope for human error. Why…
ContinueAdded by Adam Fleming on August 7, 2017 at 5:40am — No Comments
The role of validation in HACCP
The Hazard Analysis and Critical Control Point (HACCP) is a process control system that is aimed at identifying the points or areas at which hazards (dangers) may arise in the food chain. It prescribes strict measures for manufacturers and transporters of food products to prevent contamination and the resultant hazards. Control of hazards in the food chain has always been a need, but HACCP assumes added significance in today’s…
ContinueAdded by Adam Fleming on August 4, 2017 at 5:31am — No Comments
7 Keys to Compliance Excellence that Form the Foundation for Any Excellent Organization
In any industry, compliance is everything; be it highly specialized fields such as the life sciences, healthcare, medical devices or non-technological areas such as food and clothing. Why do companies need to be compliant? It is simply because being in compliance with the required regulatory requirements helps the organization to achieve the most positive outcomes needed for a business:
- It earns them a good reputation
- Increases stakeholder and customer…
Added by Adam Fleming on August 3, 2017 at 5:48am — No Comments
MS Excel can be a wonderful tool for carrying out umpteen functions
Mastering MS Excel formulas and functions can make a miracle out of this program. When used optimally, MS Excel can be a wonderful tool for carrying out umpteen functions and optimizing work related to a number of departments. For example, the Accounts Department can do a number of important functions such as loan repayment calculation, generating a profit and loss statement, solve complex mathematical and engineering problems, and carry out anything that involves addition, subtraction,…
ContinueAdded by Adam Fleming on August 2, 2017 at 5:35am — No Comments
Understanding HACCP and risk based HACCP
Hazard Analysis and Critical Control Point (HACCP) is an important system aimed at bringing down the risk of safety hazards found in food consumed all over the world. It is an internationally recognized system. When it was enacted in January 2011 as part of the FDA’s Food Safety Modernization Act; it was considered a very sweeping piece of legislation for the food industry.
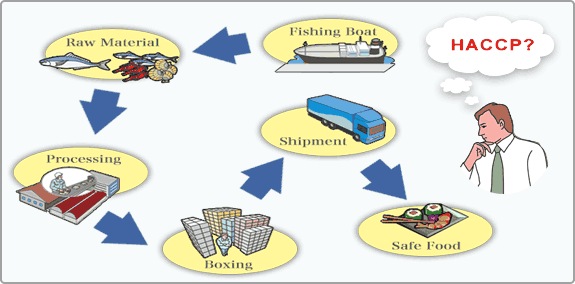
Identification and control of…
ContinueAdded by Adam Fleming on August 1, 2017 at 5:39am — No Comments
Ways of getting the PREDICT, ACE and the HTS right to smoothen shipping
In September 2014, the FDA deployed a new risk-based screening tool for imports called the Predictive Risk-based Evaluation for Dynamic Import Compliance Targeting (PREDICT). The main aim of PREDICT was bringing about improvement in screening and targeting of adulterated or misbranded goods or those that flout any of the FDA’s rules. The FDA seeks to bring this about by doing away with its legacy electronic system, OASIS’ admissibility screening function.
PREDICT is an important tool…
ContinueAdded by Adam Fleming on July 31, 2017 at 5:28am — No Comments
Sources of contamination that exist in a clean room environment
Aseptic technique is one of the methods used in eliminating or at least minimizing contamination in pathogens. It is also used to make compounding sterile products. Sterilized equipment, sterile apparel, high degree of processing, and cleaning on a continuous basis make up the important procedures used in aseptic technique.
The main aim of aseptic technique in cleanrooms is to ensure that the sterile product is sterile, safe and effective. Ensuring this is all the more important for…
ContinueAdded by Adam Fleming on July 21, 2017 at 6:08am — No Comments
Understanding opportunities in NAFTA
The North American Free Trade Agreement (NAFTA) came into being on the first day of 1994. While the US and Canada had been having free trade agreements that go back at least three decades culminating in the US-Canada Free Trade Agreement of January 1, 1989; NAFTA became a reality following Canada’s entry in 1991 into the bilateral talks between the US and Mexico, creating the trilateral FTA.
One of the primary objectives of the NAFTA was the elimination of a number of duties and…
ContinueAdded by Adam Fleming on July 20, 2017 at 5:26am — No Comments
Latest Blog Posts
- ISO 14001: What is Emergency Preparedness and Response?
- ISO 45001: What is Emergency Preparedness and Response?
- The Use of Statistical Process Control (SPC) Using Control Charts to Maintain Compliance in the Laboratory
- ISO 45001: What is Emergency Preparedness and Response?
- Supplier Auditing for Medical Device Companies
- Excel Spreadsheets - Step-By-Step Instructions for Ensuring Data Integrity
- Production and Process Controls for Medical Device Companies
Most Popular Blog Posts
- 3-Hour Virtual Seminar on FDA's section 804 Drug and 801(d)(1)(B) Biologic Importation Programs
- Metrology: Statistical Analysis of Measurement Uncertainty
- Supplier Auditing for Medical Device Companies
- Understanding normality tests and normality transformations
- You Will Never Believe These (Bizarre) Truth Behind Food And Beverages
- Death by CAPA - Does your CAPA Program need a CAPA?
- The Attribute Agreement Analysis
© 2024 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()