The Cutting Edge of Medical Technology Content, Community & Collaboration
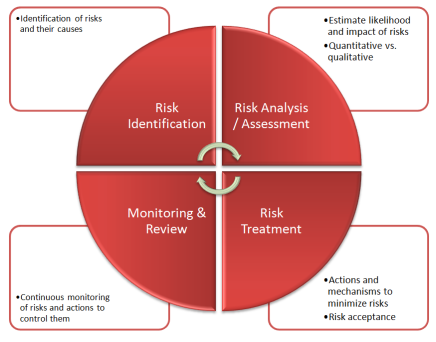
Overview of this session Product Risk Management is a critical aspect of ensuring medical devices are safe and effective for intended uses. This course will help you understand the regulatory requirements, including ISO14971, and how to create processes and procedures to implement them.
You’ll learn techniques that can help you identify hazards and potential harms. You’ll learn how to mitigate risk and effectively monitor risk to ensure your customers receive safe and effective products. A rigorous risk management process can prevent serious problems and costs for your company.
 https://compliance4all14.files.wordpress.com/2017/07/risk-management-definition.png?w=872&h=684 872w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 150w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 300w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 768w" sizes="(max-width: 436px) 100vw, 436px" width="436" height="342" />
https://compliance4all14.files.wordpress.com/2017/07/risk-management-definition.png?w=872&h=684 872w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 150w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 300w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 768w" sizes="(max-width: 436px) 100vw, 436px" width="436" height="342" />
In this webinar we’ll cover:
- Overview and Definitions
- FDA Expectations
- ISO14971 Regulation
- Linkages to Design Controls, Production Controls, Investigations, and CAPA
- Risk Management throughout the product lifecycle
- Common mistakes
- Best Practices
Why should you have to Attend The cost of poor product risk management can be staggering. Complaints, Medical Device Reports, recalls, and serious adverse impact to your customers can all result from poor risk management. And these types of problems expose you to regulatory inspections and citations. Many companies have even experienced class action law suits because of product quality issues. An effective program of risk management can help you proactively identify and mitigate product risks. A good risk management process can help you methodically identify, mitigate, and monitor risk throughout the product life-cycle.
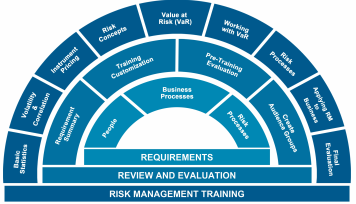
These are the areas covered by the speaker Susanne Manz
- Overview of the ISO14971 standard as it applies to medical device companies
- Integrating the new standard with ISO 13485 as part of your overall QMS
- Conducting a review of the intended use of your device
- Stages of Risk Management as well as Tools and Techniques
- Identifying hazards in your product or production process, and estimating their severity
- Judging the probability that harm may occur from those hazards
- How to control those risks and monitor the effectiveness of the controls put in place
By login with this Risk Management Techniques for Medical Devices
Which are the experts benefited by this session are as shown in the below
 https://compliance4all14.files.wordpress.com/2017/07/risk-training.png?w=712&h=408 712w, https://compliance4all14.files.wordpress.com/2017/07/risk-training.... 150w, https://compliance4all14.files.wordpress.com/2017/07/risk-training.... 300w" sizes="(max-width: 356px) 100vw, 356px" width="356" height="204" />
https://compliance4all14.files.wordpress.com/2017/07/risk-training.png?w=712&h=408 712w, https://compliance4all14.files.wordpress.com/2017/07/risk-training.... 150w, https://compliance4all14.files.wordpress.com/2017/07/risk-training.... 300w" sizes="(max-width: 356px) 100vw, 356px" width="356" height="204" />
- Design Engineer
- Manufacturing Engineer
- Quality Engineer
- R&D Personnel
- R&D Project Managers
- Quality Managers
- Auditors
- Regulatory Affairs Specialist
- R&D Manager
Susanne Manz is an accomplished leader in the medical device industry with emphasis on quality, compliance, and six sigma. She has an extensive background in quality and compliance for medical devices from new product development, to operations, to post-market activities. While at GE, J&J, and Medtronic, Susanne worked in various world-wide roles including Executive Business Consultant, WW Director of Quality Engineering and, Design Quality, and Director of Corporate Compliance. Susanne has a BS in Biomedical Engineering and an MBA from the University of NM.
Views: 7
Comment
© 2025 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()
You need to be a member of MedTech I.Q. to add comments!
Join MedTech I.Q.