The Cutting Edge of Medical Technology Content, Community & Collaboration
The 60601-1 is a standard that relates to the safety of medical electrical equipment. This harmonized standard is recognized by most countries around the world. Existing and new medical devices have to comply with the requirements set out in IEC 60601-1.
The IEC 60601-1 is going through its latest revision, its 3rd edition, which came into effect in June 2012. The regulatory agencies of various countries that have adapted this standard are in various stages of implementation. Documentation and certification for IEC 60601-1 is to be done in stages at various dates, which will go up to 2018.
 https://compliance4all14.files.wordpress.com/2017/07/60601.jpg?w=150 150w, https://compliance4all14.files.wordpress.com/2017/07/60601.jpg?w=300 300w" sizes="(max-width: 448px) 100vw, 448px" />
https://compliance4all14.files.wordpress.com/2017/07/60601.jpg?w=150 150w, https://compliance4all14.files.wordpress.com/2017/07/60601.jpg?w=300 300w" sizes="(max-width: 448px) 100vw, 448px" />
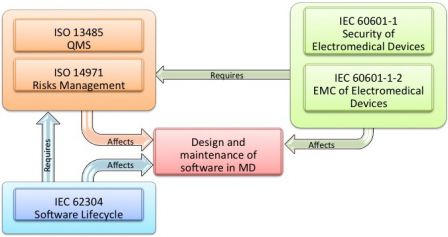
Major product safety changes brought about by the 3rd edition
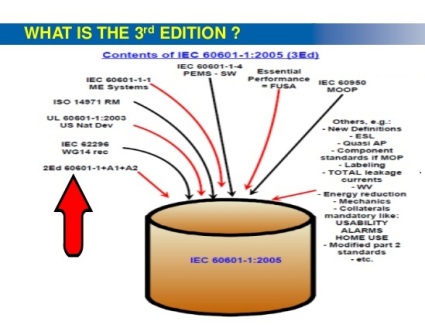
Through the publication of Amendment I, IEC 60601-1 brings in around 20 new requirements and some 60 modifications to the existing requirements. The major change IEC 60601-1’s latest version, 3.1 of the 3rd edition brings is that it makes a risk management file and process that aligns with ISO 14971 mandatory for manufacturers of medical electrical equipment. The ISO 14971 is the global standard that applies to risk management in medical devices.
The IEC 60601-1 3.1 Edition also requires design review and third party approval for medical devices. In addition, this version has also modified requirements in relation to these among many other areas:
- Essential Performance
- Documentation
- Humidity
- Marking and Labeling
- Temperature Testing
- Programmable Electrical Medical Systems (PEMS)
- Mechanical Hazards
- Electrical Hazards
- Construction
 https://compliance4all14.files.wordpress.com/2017/07/risk-management-in606011-14-638.jpg?w=150&h=118 150w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 300w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 549w" sizes="(max-width: 425px) 100vw, 425px" width="425" height="335" />
https://compliance4all14.files.wordpress.com/2017/07/risk-management-in606011-14-638.jpg?w=150&h=118 150w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 300w, https://compliance4all14.files.wordpress.com/2017/07/risk-managemen... 549w" sizes="(max-width: 425px) 100vw, 425px" width="425" height="335" />
Stay clear of the complexity
Want to understand the ways by which the new IEC 60601-1 version works for your organization? Want to clear the confusions regarding implementation of the new IEC 60601-1 standard and gain acceptance by meeting compliance requirements set out by the various regulatory agencies? A webinar that is being organized by Compliance4All, a leading provider of professional trainings for all the areas of regulatory compliance will provide all the answers.
The guru of IEC 60601-1, Leonard Eisner, will be the speaker at this highly insightful session. The Founder and Principal Consultant at Eisner Safety Consultants, which specializes in helping medical device manufacturers through product safety, international regulatory (FDA 510(k), CE Mark, Canadian Medical Device Regulations (CMDR), etc.) and quality system processes; Leo, a licensed professional engineer in safety engineering, a Notified Body and Quality System auditor/technical reviewer, an expert in product safety for medical electrical devices (IEC 60601 series of standards) and an expert in CE marking for the medical device EU Directives, has helped countless clients through the Product Safety and Regulatory maze over his career.
To register for this highly interesting and relevant session, please visit 16 Steps to Get Approval to IEC 60601-1
Learning from others’ mistakes
Leo will help participants accelerate their time to market. He will show how to follow the steps and hasten the speed at which they can obtain their product certification to IEC 60601-1 series of standards. All the expectations for product testing set out by certification agencies to the IEC 60601-1 series of standards on medical electrical equipment and systems, such as UL, TUV, BSI, Intertek, etc., will be explained.
An important element of the learning at this webinar is the ways by which to learn from the mistakes Leo has seen others make that have gone on to slow their certification process. He will show the participants what proactive steps they can take to avoid these mistakes and ensure that their product doesn’t need to get redesigned after testing starts.
 https://compliance4all14.files.wordpress.com/2017/07/isoiec80001-do-we-need-another-standard-14-638.jpg?w=150 150w, https://compliance4all14.files.wordpress.com/2017/07/isoiec80001-do... 300w" sizes="(max-width: 604px) 100vw, 604px" />
https://compliance4all14.files.wordpress.com/2017/07/isoiec80001-do-we-need-another-standard-14-638.jpg?w=150 150w, https://compliance4all14.files.wordpress.com/2017/07/isoiec80001-do... 300w" sizes="(max-width: 604px) 100vw, 604px" />
Leo will cover the following areas at this webinar:
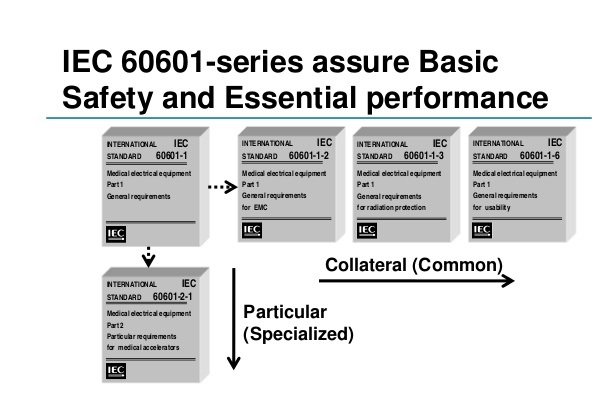
- Learn about the scope of the IEC 60601-1 standard & if it applies to your product. Also, what are the Collateral Standards (IEC 60601-1-X) and Particular Standards are about
- What you need to know to classify your products to the IEC 60601-1 series
- What is an isolation diagram and how does that help me with my design?
- Determine the applicable tests for your device
- What are the marking and labeling requirements for the device?
- Know your critical components
- What pre-tests to run and what’s not worth pre-testing?
Views: 33
Comment
© 2026 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()
You need to be a member of MedTech I.Q. to add comments!
Join MedTech I.Q.