 This is a great deal. What else does one call this offer $10 deal to enhance professional skill from GlobalCompliancePanel? Wait. We are talking about $10, but did we tell you what you get for $10? Did you think it is the cost of registration for the event?…
This is a great deal. What else does one call this offer $10 deal to enhance professional skill from GlobalCompliancePanel? Wait. We are talking about $10, but did we tell you what you get for $10? Did you think it is the cost of registration for the event?…
The Cutting Edge of Medical Technology Content, Community & Collaboration
All Blog Posts Tagged 'FDA' (145)
 Special Deal 2017 - JUST $10 for Healthcare Compliance Course Attendees!
Special Deal 2017 - JUST $10 for Healthcare Compliance Course Attendees!
For regulatory professionals who work in the field of healthcare, compliance with the regulatory requirements is a must. We are all aware of it. But if the cost of healthcare compliance courses is causing you to put off your trainings, GlobalCompliancePanel has the answer: Its special deal in 2017 for healthcare compliance course attendees makes trainings in healthcare compliance available for $10. Yes, you’ve read that right. All it takes is $10 to enroll for a course in healthcare…
ContinueAdded by John Robinson on August 22, 2017 at 7:15am — No Comments
 Life Sciences Course Offer 2017 by GlobalCompliancePanel Just $10
Life Sciences Course Offer 2017 by GlobalCompliancePanel Just $10
Did you know that an online life sciences course offer 2017 by GlobalCompliancePanel @ 10$ is on? Want me repeat this to confirm its veracity? Yes. It is true, and it is real. An online life sciences course offer 2017 by GlobalCompliancePanel @ 10$ is underway! But is it possible for anyone to offer online life sciences…
Added by John Robinson on August 22, 2017 at 7:12am — No Comments
 Risk Management Webinars Online - Just Pay @ $10
Risk Management Webinars Online - Just Pay @ $10
Risk Management Webinars Online - Just Pay @ $10. This is not the catchphrase for an ad that is showing up the wrong product by mistake. You could be forgiven for thinking so, for it is natural and predictable to mistake these catchy words for being ads for some daily use items. But then, these words are not the slogans for products or services that are meant for daily use.…
Added by John Robinson on August 22, 2017 at 7:00am — No Comments
 Don’t miss out! Offer @ $10 - Online Professional Courses
Don’t miss out! Offer @ $10 - Online Professional Courses
Added by John Robinson on August 22, 2017 at 5:56am — No Comments
 Relationship Between an EM Excursion Program and CAPA
Relationship Between an EM Excursion Program and CAPA
The FDA’s regulations on cleanroom environmental monitoring (EM) are based on the assumption that any person who enters a cleanroom is likely and almost certain to have brought contaminants in one or another form with him. The FDA’s regulations on cleanroom environmental monitoring are built on the thinking that microorganisms can assail even the cleanest of systems, which is why its regulations on cleanroom…
ContinueAdded by Adam Fleming on August 9, 2017 at 5:39am — No Comments
 Actions for Noncompliance of cGMPs in the Quality Control Laboratory
Actions for Noncompliance of cGMPs in the Quality Control Laboratory
Quality controls in laboratories are a major area for which the FDA issues 483’s. A laboratory is the venue for many activities, all of them of varying importance to the product. When controls in laboratories are not up to the standard, such a laboratory could produce products that do not meet quality and processes expectations, and hence invite 483’s.

Issues with drug quality, drug integrity and data integrity, as well as data fabrication and human errors…
ContinueAdded by Adam Fleming on August 8, 2017 at 5:36am — No Comments
 Ways of getting the PREDICT, ACE and the HTS right to smoothen shipping
Ways of getting the PREDICT, ACE and the HTS right to smoothen shipping
In September 2014, the FDA deployed a new risk-based screening tool for imports called the Predictive Risk-based Evaluation for Dynamic Import Compliance Targeting (PREDICT). The main aim of PREDICT was bringing about improvement in screening and targeting of adulterated or misbranded goods or those that flout any of the FDA’s rules. The FDA seeks to bring this about by doing away with its legacy electronic system, OASIS’ admissibility screening function.
PREDICT is an important tool…
ContinueAdded by Adam Fleming on July 31, 2017 at 5:28am — No Comments
 How to create processes and procedures to implement them?
How to create processes and procedures to implement them?
Product Risk Management is a critical aspect of ensuring medical devices are safe and effective for intended uses. This course will help you understand the regulatory requirements, including ISO14971.
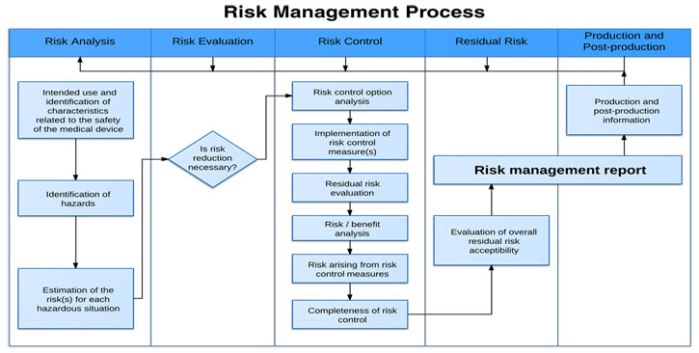
You’ll learn techniques that can help you identify hazards and potential harms. You’ll learn how to mitigate risk and effectively monitor risk to ensure your customers receive safe and effective products. A rigorous…
ContinueAdded by Adam Fleming on July 19, 2017 at 5:47am — No Comments
 How to control those risks and monitor the effectiveness of the controls put in place
How to control those risks and monitor the effectiveness of the controls put in place
Overview of this session Product Risk Management is a critical aspect of ensuring medical devices are safe and effective for intended uses. This course will help you understand the regulatory requirements, including ISO14971, and how to create processes and procedures to implement them.
You’ll learn techniques that can help you identify hazards and potential harms. You’ll learn how to mitigate risk and effectively monitor risk to ensure your customers receive safe and effective…
ContinueAdded by Adam Fleming on July 13, 2017 at 5:43am — No Comments
 FDA holds Cosmetic Manufacturers & distributors responsible for the safety and quality of their Cosmetics
FDA holds Cosmetic Manufacturers & distributors responsible for the safety and quality of their Cosmetics
Testing cosmetics before the FDA does it is a sound business strategy for cosmetic manufacturers. This is why: Cosmetic manufacturers and distributors are responsible for upholding the quality and safety standards set out by the FDA. This is a legal requirement on the part of players in the cosmetics industry. In order to ensure compliance…
Added by Adam Fleming on June 7, 2017 at 6:11am — No Comments
 Implementing the Final FDA FSMA Rules on the Sanitary Transportation of Human and Animal Foods
Implementing the Final FDA FSMA Rules on the Sanitary Transportation of Human and Animal Foods
The FDA’s Food Safety Modernization Act (FSMA) rule on Sanitary Transportation of Human and Animal Food, which was passed on April 6, 2016, has now become final. This is the latest in a series of as many as seven rules that have been getting legislated from January 2013 with the intention of creating a modern, risk-based framework that ensures food…
Added by Adam Fleming on May 30, 2017 at 5:54am — No Comments
 Why IEC 60825 certification cannot be substituted for 21CFR1040
Why IEC 60825 certification cannot be substituted for 21CFR1040
With a few exceptions, it is necessary for manufacturers, system integrators and importers of lasers or laser containing products to implement best practices for compliance with FDA 21 CFR 1040. This is because The Center for Devices and Radiological Health (CDRH) requests documentation using the current version of their Form 3632 Guide for Preparing Product Reports for Lasers or Products Containing Lasers for laser self-certification submittals, which have to comply with…
Added by Adam Fleming on May 24, 2017 at 6:31am — No Comments
 Seminar Calendar of Upcoming Courses - June to July - 2017
Seminar Calendar of Upcoming Courses - June to July - 2017
GlobalCompliancePanel’s seminars are a wonderful opportunity for professionals in the regulatory compliance areas to understand the latest happenings and updates in the…
ContinueAdded by John Robinson on May 24, 2017 at 5:51am — No Comments
 Webinar Calendar of Upcoming Courses - May to Jun 2017
Webinar Calendar of Upcoming Courses - May to Jun 2017

Below is the event description content:
Compliance4All webinars are just what professionals in the regulatory
compliance areas need for scaling up in their careers. With a collection of
the most erudite experts on regulatory compliance being available at a click
in the comfort of your preferred location; regulatory compliance could not
get any simpler and effective!
Compliance4All’s experts help you unravel all the knowledge you need…
Added by Adam Fleming on May 22, 2017 at 7:53am — No Comments
 How does the FDA scrutinize Promotion and Advertising Practices?
How does the FDA scrutinize Promotion and Advertising Practices?
The FDA has strict requirements on the way promotion and advertising practices are to be implemented by the industries that it regulates. Section 906 of the Food and Drug Administration Amendments Act (FDAAA), which came into effect in 2008 and amended the section that pertained to this topic previously, namely Section 502(n) of the Federal Food, Drug, and Cosmetic Act (FDCA); now requires that published Direct to Consumer (DTC) advertisements for prescription drugs should…
Added by Adam Fleming on May 11, 2017 at 6:23am — No Comments
 Article on “Human factors as a factor in medical devices 2017”
Article on “Human factors as a factor in medical devices 2017”
Added by John Robinson on May 10, 2017 at 6:27am — No Comments
 The ingredients of a truly effective internal audit program
The ingredients of a truly effective internal audit program
A n internal audit program of an organization’s Quality Management Systems is a core requirement from both the ISO and the FDA. At its barest, an internal audit system can be understood to be an assessment or inspection of an organization’s Quality System. Audits are a very beneficial and positive tool for continually ensuring that an organization’s internal management…
n internal audit program of an organization’s Quality Management Systems is a core requirement from both the ISO and the FDA. At its barest, an internal audit system can be understood to be an assessment or inspection of an organization’s Quality System. Audits are a very beneficial and positive tool for continually ensuring that an organization’s internal management…
Added by John Robinson on May 3, 2017 at 5:55am — No Comments
 Article on “A Tour of the FDA”
Article on “A Tour of the FDA”
Added by John Robinson on April 27, 2017 at 5:50am — No Comments
 Applied Statistics for FDA Process Validation 2017
Applied Statistics for FDA Process Validation 2017
Added by John Robinson on April 12, 2017 at 5:30am — No Comments
 Right now Medical device hazard analysis, the core of medical devices
Right now Medical device hazard analysis, the core of medical devices
Medical device hazard analysis is of vital importance to a medical device. Medical device hazard analysis is at the heart of medical devices because if the device is not analyzed thoroughly for the hazard, or danger, that it poses, it is likely to cause problems of any kind to the user. Many a time, it becomes a matter of life and death. This is why medical device hazard analysis is of foremost importance.…
Added by Adam Fleming on March 27, 2017 at 8:31am — No Comments
Latest Blog Posts
- Consultation about FDA 510 k clearance and submission
- Production and Process Controls for Medical Device Companies
- Design History Files (DHF), Device Master Records (DMR), Device History Records (DHR), Technical Files , Design Dossiers - The Requirements
- Electronic Records & Electronic Signatures; 21 CFR Part 11; Basic Concepts
- Dietary Supplements CGMPS - 21 CFR 111 Compliance
- Validation of HPLC/UPLC Methodologies
- Validation of HPLC/UPLC Methodologies
Most Popular Blog Posts
- What is the legal language of the FDA form 1572 or Device equivalent?
- IT’S A NO BRAINER! Action needed to stop children being exposed to chemicals that harm their brain development!
- A Process Approach to Quality Management Systems
- FDA Warning Letters – an understanding
- Orkambi Reduces Main Biomarker of CF, Vertex Says in Updated Results on Four Therapies
- The use of Applied Statistics for FDA Process Validation
- Understanding Kidney Tumours
Monthly Archives
2024
- May (1)
2022
2020
2019
2018
- December (5)
- November (8)
- October (8)
- September (7)
- August (8)
- July (9)
- June (3)
- April (7)
- March (7)
- February (21)
- January (32)
2017
- December (11)
- November (39)
- October (64)
- September (60)
- August (34)
- July (30)
- June (22)
- May (40)
- April (16)
- March (12)
- February (18)
- January (15)
2016
2015
2014
2013
2012
- October (3)
- September (1)
- August (9)
- July (8)
- June (8)
- May (11)
- April (6)
- March (11)
- February (1)
- January (6)
2011
- December (2)
- November (3)
- October (7)
- September (3)
- July (4)
- June (4)
- April (7)
- March (4)
- February (3)
- January (7)
2010
- December (8)
- November (11)
- October (6)
- September (11)
- August (18)
- July (28)
- June (24)
- May (10)
- April (31)
- March (44)
- February (45)
- January (85)
2009
- December (104)
- November (63)
- October (44)
- September (68)
- August (53)
- July (37)
- June (50)
- May (43)
- April (63)
- March (106)
- February (36)
- January (15)
2008
1999
- November (5)
© 2024 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()



