The Cutting Edge of Medical Technology Content, Community & Collaboration
Of late, the FDA has been turning on the heat on manufacturers in the FDA-regulated industries that violate its regulations. It has a penchant for going after manufacturing facilities that show laxity in implementing current Good Manufacturing Practices (cGMP). This ardor is understandable. cGMP violations affect the quality of the product; hence the strictness, considering that it is patients who consume these products.

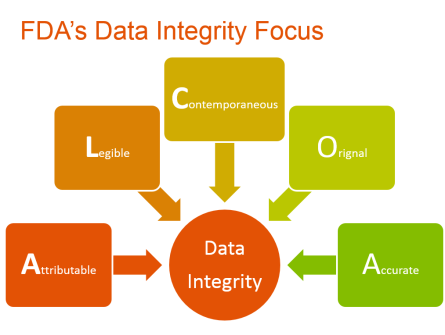
There are ways of ensuring that the product meets the acute scientific requirements set out for it at all stages, from the beginning till the end. Data integrity is the most important of these. As the term suggests, data integrity is the assurance that there is complete accuracy, security, reliability and completeness of the records relating to the product and its processes.
Data integrity comes about by tightening cGMP requirements
In the FDA’s line of thinking, the way of ensuring uncompromising quality of a product is by tightening its laws on cGMP and implementing them. The FDA believes that only extremely stringent actions on its part help manufacturers meet regulatory requirements and give patients the assurance that the products they use meet quality standards.
Among the many mechanisms that the FDA has undertaken towards ensuring this, requirements relating to data integrity rank high. Data integrity is critical to ensure the quality, safety, and efficacy of products in the FDA-regulated industries. These include biologics, pharmaceuticals, life sciences and medical devices, among others.
What happens when there is no data integrity?
Issues relating to data integrity have been high on the list of items that the FDA finds out during its inspections. When companies fail to meet the FDA’s data integrity expectations and requirements; data integrity is compromised. When this happens, many batches of finished goods that do not comply with regulatory authorization terms get manufactured. The FDA prohibits such lots and batches for release for sale.
Lack of data integrity is viewed very seriously by the FDA. It considers this as falsification or breach of data and passes heavy strictures on such companies. It initiates a series of penal actions on such companies after comprehensive investigations show lack of data integrity. These are some of the FDA actions that could accrue from data integrity breach:
- 483’s
- Consent decrees
- Import alerts
- Warning Letters.
Another additional enforcement action that could result from this scenario is carrying out a full risk assessment to establish the potential of the data integrity-deficient drug to cause problems for patients who consume their products. And then, the FDA could also suggest management actions that seek to correct the issues arising out of data integrity breach.
Complete understanding of data integrity
A look at the grave consequences arising out of the inability to meet the FDA’s requirements on data integrity points to the absolute need for organizations in any FDA-regulated industry to get a complete grasp of all aspects of data integrity, which will help it to avoid FDA citations. This learning will be imparted at a valuable webinar that Compliance4All, a leading provider of professional trainings for all the areas of regulatory compliance, is organizing.
Danielle DeLucy, who owns ASA Training and Consulting, LLC which provides Pharmaceutical and Biologics based companies with training and quality systems assistance in order to meet Regulatory compliance, will be the speaker at this webinar. Want to hear from her on how to implement steps for ensuring data integrity? Then, please enroll for this webinar by visiting Management of GMP Data Integrity
Total grasp of cGMP requirements on data integrity
21 CFR Part 11 is only a part of data integrity and security breaches. Data integrity and security breaches can also involve severe cGMP violations. This webinar’s speaker will analyze these and offer information about practices that the participants can review and implement at their own site and identify gaps in their practices. The importance of data integrity in assuring the quality of raw materials, in-process materials and finished goods in the cGMP for FDA-regulated industries will be explained.
These areas will be covered at this webinar:
- Discover the Criteria for Data Integrity
- Recognize what needs to be addressed to ensure Data Integrity within a regulated GxP Laboratory
- Learn about approaches to improve Data Integrity in a Laboratory Environment.
Professionals in the regulated industries, such as Site Quality Operations Managers, Quality Assurance Personnel, Plant Managers and Supervisors, Manufacturing Superintendents and Managers and Regulatory Affairs Managers will find this webinar on implementation and management of cGMP data integrity highly worthwhile.
Views: 14
Comment
© 2025 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()
You need to be a member of MedTech I.Q. to add comments!
Join MedTech I.Q.