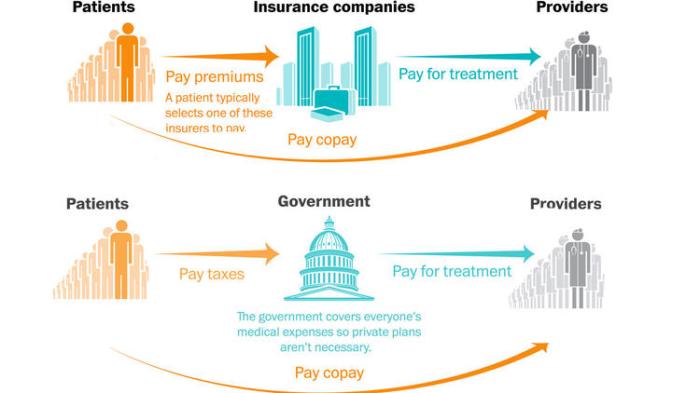
The US economy’s appetite for ingesting food imports is phenomenal, to use a mild term. How else does one describe an economy that takes in $49 billion a year? Considering that the FDA is the sole regulating agency that is tasked with overseeing imports of this magnitude, who can envy its responsibility? This is not all that the FDA does: its allied regulatory agencies monitor about half a million facilities in the US and abroad.
Since foods from…

 What the FDA’s recent guidance documents covering GMP requirements for Phase I products have done is to significantly reduce a few of the complexities that early phase products are typically against. These guidance documents are in addition to those that cover the
What the FDA’s recent guidance documents covering GMP requirements for Phase I products have done is to significantly reduce a few of the complexities that early phase products are typically against. These guidance documents are in addition to those that cover the