 Software project management has an important tool in the Agile methodology. The Agile methodology developed as a product of the gradual efforts at arriving at a team…
Software project management has an important tool in the Agile methodology. The Agile methodology developed as a product of the gradual efforts at arriving at a team…
The Cutting Edge of Medical Technology Content, Community & Collaboration
All Blog Posts Tagged 'medical' (159)
 It is important and necessary to document Software for FDA Submissions
It is important and necessary to document Software for FDA Submissions
Added by John Robinson on August 7, 2017 at 6:17am — No Comments
 The Ultimate Guide To Design Controls For Medical Device Startups
The Ultimate Guide To Design Controls For Medical Device Startups
Added by John Robinson on July 27, 2017 at 6:09am — No Comments
 The Ultimate Guide To Design Controls For Medical Device Startups
The Ultimate Guide To Design Controls For Medical Device Startups
Added by John Robinson on July 27, 2017 at 6:08am — No Comments
 Quality Assurance Auditing for FDA-regulated industries
Quality Assurance Auditing for FDA-regulated industries
An effective audit constitutes the heart of an effective Quality System. The FDA and other regulatory agencies have emphasized this principle time and again. The purpose of an audit program is to ensure proper and thorough compliance with the guidelines set out by the regulatory agencies. A…
Added by John Robinson on July 26, 2017 at 5:52am — No Comments
 Implementing ICH guidelines-compliant validation
Implementing ICH guidelines-compliant validation
Professionals in the field of statistical analysis need to possess clarity on understanding and interpreting statistical concepts used to investigate quantitative ICH Guidelines, such as:
- Analytical methods validation
- Procedures and acceptance criteria in calibration limits…
Added by John Robinson on July 24, 2017 at 6:07am — No Comments
 An integrated approach is needed for Complaints Handling, Adverse Event Reporting, and Recalls
An integrated approach is needed for Complaints Handling, Adverse Event Reporting, and Recalls
The fact that medical device manufacturers work and operate in various regulatory systems whose requirements are different and not always consistent with each other is recognized and empathized by the…
Added by John Robinson on July 21, 2017 at 5:56am — No Comments
 How to create processes and procedures to implement them?
How to create processes and procedures to implement them?
Product Risk Management is a critical aspect of ensuring medical devices are safe and effective for intended uses. This course will help you understand the regulatory requirements, including ISO14971.
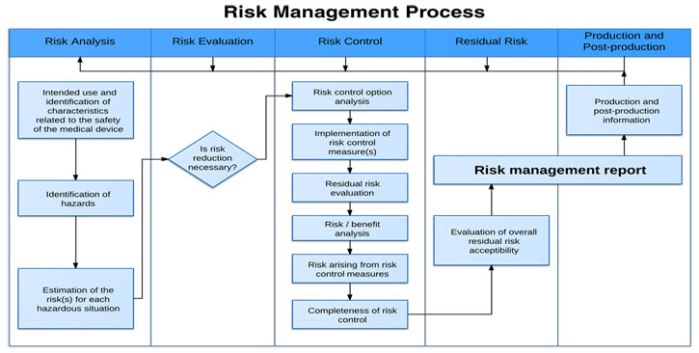
You’ll learn techniques that can help you identify hazards and potential harms. You’ll learn how to mitigate risk and effectively monitor risk to ensure your customers receive safe and effective products. A rigorous…
ContinueAdded by Adam Fleming on July 19, 2017 at 5:47am — No Comments
 Steps to IEC 60601-1 approval
Steps to IEC 60601-1 approval
The 60601-1 is a standard that relates to the safety of medical electrical equipment. This harmonized standard is recognized by most countries around the world. Existing and new medical devices have to comply with the requirements set out in IEC 60601-1.
The IEC 60601-1 is going through its latest revision, its 3rd edition,…
ContinueAdded by Adam Fleming on July 14, 2017 at 6:27am — No Comments
 The ways of applying ISO 14971, IEC 62304 and IEC62366-1 to medical device software
The ways of applying ISO 14971, IEC 62304 and IEC62366-1 to medical device software

Diligence, complete and proper examination and assessment of the gaps, and correction of the gaps right from the very start of product development are the core characteristics that need to go into implementing risk management of software used in medical devices.
These are the reasons for it:
- Lack of proper and complete implementation and gaps in them lead to major drawbacks such as production delays or…
Added by John Robinson on July 13, 2017 at 6:47am — No Comments
 How to control those risks and monitor the effectiveness of the controls put in place
How to control those risks and monitor the effectiveness of the controls put in place
Overview of this session Product Risk Management is a critical aspect of ensuring medical devices are safe and effective for intended uses. This course will help you understand the regulatory requirements, including ISO14971, and how to create processes and procedures to implement them.
You’ll learn techniques that can help you identify hazards and potential harms. You’ll learn how to mitigate risk and effectively monitor risk to ensure your customers receive safe and effective…
ContinueAdded by Adam Fleming on July 13, 2017 at 5:43am — No Comments
 Do human factors matter in medical devices?
Do human factors matter in medical devices?

Is there a relationship between medical devices and human factors? This is a question that is seriously worth exploring. According to the ANSI/AAMI HE75:2009 document, human factors is an endeavor for optimizing the production of devices, systems, and many others concerned with them through the use of emotional, intellectual,…
Added by John Robinson on July 12, 2017 at 6:21am — No Comments
 Advantages and disadvantages of Electronic Health Records
Advantages and disadvantages of Electronic Health Records
Electronic Health Records (EHR) or electronic medical records (EMR), as they are called, are of enormous use in the fields of healthcare and medical sciences. They have a number of features that enable the patient; the medical professional and the healthcare provider have complete and unimaginably easy access to all important records that relate to the patient.
A direct result of the…
ContinueAdded by Roger Steven on June 29, 2017 at 7:52am — No Comments
 Why does FDA think they can Regulate them, and why do others think they Cannot
Why does FDA think they can Regulate them, and why do others think they Cannot
This presentation will review the legal definition of medical device, and how it is applied by FDA to in vitro diagnostic tests. It will review the history of FDA interest in LDTs, and will describe the current situation with respect to Laboratory-developed tests. It will describe tests which have been cleared by FDA and those which have attempted clearance but not been cleared. It will discuss possible future actions by FDA and by the US laboratory community and assess their…
ContinueAdded by Adam Fleming on June 29, 2017 at 5:21am — No Comments
 Documenting Software for FDA Submissions
Documenting Software for FDA Submissions

The Agile methodology is an important tool for software project management. It emerged out of the gradual efforts at arriving at a team based methodology of iterative software development. Its close association with software makes it as suitable to this field as Lean is to manufacturing.…
Added by John Robinson on June 27, 2017 at 6:15am — No Comments
 All about biosimilars –from development to registration
All about biosimilars –from development to registration

Biosimilars can be described as near-copies of an original pharmaceutical product that another company may have manufactured. These products are versions of an original or innovative product, but are officially approved.
There is a misconception that they are similar to or are the same as generics, but this is not so in…
Added by John Robinson on June 27, 2017 at 6:01am — No Comments
 Validation the complies with ICH guidelines
Validation the complies with ICH guidelines
Added by John Robinson on June 5, 2017 at 7:26am — No Comments
 Meeting labeling requirements of various drug products
Meeting labeling requirements of various drug products
Both the FDA and the European Medicines Agency (EMA) have regulations that cover the labeling requirements of both prescription and over-the-counter (OTC) drugs, cosmetics, generics, medical devices, nutraceuticals and other related products. These regulations have to be strictly complied with. 21 CFR under its various parts, the Federal Food, Drug, and…
Added by Adam Fleming on May 19, 2017 at 6:17am — No Comments
 Regulations governing how combination products are regulated
Regulations governing how combination products are regulated
The knowledge of combination products and their regulations is essential for regulatory professionals. This is because of the high proportion of combination products in the market, as well as the array of regulations that govern them.
21 CFR 3.2 (e) has a complete definition of a combination product. A combination product is one…
Added by Adam Fleming on May 17, 2017 at 6:40am — No Comments
 Marketing and promotion of drugs and medical devices
Marketing and promotion of drugs and medical devices
Added by John Robinson on May 16, 2017 at 6:22am — No Comments
 Article on “Human factors as a factor in medical devices 2017”
Article on “Human factors as a factor in medical devices 2017”
Added by John Robinson on May 10, 2017 at 6:27am — No Comments
Latest Blog Posts
- What is the UKCA Mark for Medical Device?
- Production and Process Controls for Medical Device Companies
- Trial Registration and Results Reporting on ClinicalTrials.gov
- How to Find the Right “DIETARY SUPPLEMENTS” for Your Specific Product (Service)
- eCTD Submissions of IND-NDA to the US FDA, EU and Canada
- Effective Technical Writing in the Life Sciences
- The [Ultimate Guide] To Medical Device Inspection
Most Popular Blog Posts
- Medical records retention and disposition is a complex activity
- Harrisburg Health Information Exchange Submits ARRA Health IT Grant
- Medical Affairs Services: Role in Planning of New Pharmaceutical Product Launch
- NIH Challenge Grants (RFA-OD-09-003) – New Funding Opportunity for Small Businesses (due date: April 27, 2009)
- The [Ultimate Guide] To Medical Device Inspection
- What is the UKCA Mark for Medical Device?
- Things you most likely didn’t know about [OSHA Compliance]
Monthly Archives
2024
- May (1)
2022
2020
2019
2018
- December (5)
- November (8)
- October (8)
- September (7)
- August (8)
- July (9)
- June (3)
- April (7)
- March (7)
- February (21)
- January (32)
2017
- December (11)
- November (39)
- October (64)
- September (60)
- August (34)
- July (30)
- June (22)
- May (40)
- April (16)
- March (12)
- February (18)
- January (15)
2016
2015
2014
2013
2012
- October (3)
- September (1)
- August (9)
- July (8)
- June (8)
- May (11)
- April (6)
- March (11)
- February (1)
- January (6)
2011
- December (2)
- November (3)
- October (7)
- September (3)
- July (4)
- June (4)
- April (7)
- March (4)
- February (3)
- January (7)
2010
- December (8)
- November (11)
- October (6)
- September (11)
- August (18)
- July (28)
- June (24)
- May (10)
- April (31)
- March (44)
- February (45)
- January (85)
2009
- December (104)
- November (63)
- October (44)
- September (68)
- August (53)
- July (37)
- June (50)
- May (43)
- April (63)
- March (106)
- February (36)
- January (15)
2008
1999
- November (5)
© 2024 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()











