A number of reasons make cultivation of the art of effective legal writing skills for FDA submissions important. Documents that are submitted to the FDA are…
The Cutting Edge of Medical Technology Content, Community & Collaboration
July 2017 Blog Posts (30)
 Effective legal writing skills are essential for FDA submissions
Effective legal writing skills are essential for FDA submissions
Added by John Robinson on July 31, 2017 at 6:28am — No Comments
 Ways of getting the PREDICT, ACE and the HTS right to smoothen shipping
Ways of getting the PREDICT, ACE and the HTS right to smoothen shipping
In September 2014, the FDA deployed a new risk-based screening tool for imports called the Predictive Risk-based Evaluation for Dynamic Import Compliance Targeting (PREDICT). The main aim of PREDICT was bringing about improvement in screening and targeting of adulterated or misbranded goods or those that flout any of the FDA’s rules. The FDA seeks to bring this about by doing away with its legacy electronic system, OASIS’ admissibility screening function.
PREDICT is an important tool…
ContinueAdded by Adam Fleming on July 31, 2017 at 5:28am — No Comments
 Vital Stark Law considerations for physician employment agreements
Vital Stark Law considerations for physician employment agreements
Since the Affordable Care Act (ACA) –popularly called Obamacare –came into existence, there has been an exponential increase in the number of physician practice acquisitions. Some of the reasons that can be ascribed for this phenomenon are these:
- Physician practice acquisitions are an opportunity for healthcare providers in consolidating and…
ContinueAdded by Roger Steven on July 28, 2017 at 7:30am — No Comments
 The Ultimate Guide To Design Controls For Medical Device Startups
The Ultimate Guide To Design Controls For Medical Device Startups
Added by John Robinson on July 27, 2017 at 6:09am — No Comments
 The Ultimate Guide To Design Controls For Medical Device Startups
The Ultimate Guide To Design Controls For Medical Device Startups
Added by John Robinson on July 27, 2017 at 6:08am — No Comments
 Quality Assurance Auditing for FDA-regulated industries
Quality Assurance Auditing for FDA-regulated industries
An effective audit constitutes the heart of an effective Quality System. The FDA and other regulatory agencies have emphasized this principle time and again. The purpose of an audit program is to ensure proper and thorough compliance with the guidelines set out by the regulatory agencies. A…
Added by John Robinson on July 26, 2017 at 5:52am — No Comments
 The centrality of Risk Analysis to HIPAA
The centrality of Risk Analysis to HIPAA
Carrying out audits is among the most important requirements for any organization that handles Protected Health Information (PHI). HIPAA hosting providers, who implement safeguards in the manner specified in the HIPAA Privacy Rule, must be included in these audits. The indispensability of audits…
ContinueAdded by Roger Steven on July 25, 2017 at 8:00am — No Comments
 Which universities are pushing the boundaries in life sciences?
Which universities are pushing the boundaries in life sciences?
If you had to name the branch of university research that has the most tangible impact on mankind’s day-to-day activities, it is likely that the life sciences would be near the top of the list: not many days go by without the announcement of a new drug or gene discovery that has the potential to change lives or tackle disease.
Much of the best research in these fields takes place in the ultra-elite universities that excel in subjects across the…
Added by John Robinson on July 25, 2017 at 6:14am — No Comments
 Implementing ICH guidelines-compliant validation
Implementing ICH guidelines-compliant validation
Professionals in the field of statistical analysis need to possess clarity on understanding and interpreting statistical concepts used to investigate quantitative ICH Guidelines, such as:
- Analytical methods validation
- Procedures and acceptance criteria in calibration limits…
Added by John Robinson on July 24, 2017 at 6:07am — No Comments
 Sources of contamination that exist in a clean room environment
Sources of contamination that exist in a clean room environment
Aseptic technique is one of the methods used in eliminating or at least minimizing contamination in pathogens. It is also used to make compounding sterile products. Sterilized equipment, sterile apparel, high degree of processing, and cleaning on a continuous basis make up the important procedures used in aseptic technique.
The main aim of aseptic technique in cleanrooms is to ensure that the sterile product is sterile, safe and effective. Ensuring this is all the more important for…
ContinueAdded by Adam Fleming on July 21, 2017 at 6:08am — No Comments
 An integrated approach is needed for Complaints Handling, Adverse Event Reporting, and Recalls
An integrated approach is needed for Complaints Handling, Adverse Event Reporting, and Recalls
The fact that medical device manufacturers work and operate in various regulatory systems whose requirements are different and not always consistent with each other is recognized and empathized by the…
Added by John Robinson on July 21, 2017 at 5:56am — No Comments
 Understanding opportunities in NAFTA
Understanding opportunities in NAFTA
The North American Free Trade Agreement (NAFTA) came into being on the first day of 1994. While the US and Canada had been having free trade agreements that go back at least three decades culminating in the US-Canada Free Trade Agreement of January 1, 1989; NAFTA became a reality following Canada’s entry in 1991 into the bilateral talks between the US and Mexico, creating the trilateral FTA.
One of the primary objectives of the NAFTA was the elimination of a number of duties and…
ContinueAdded by Adam Fleming on July 20, 2017 at 5:26am — No Comments
 Training and Development is a highly developed and evolved, broad body of knowledge. Many employees place themselves at a disadvantage vis-à-vis their colleagues in the absence of the right professio…
Training and Development is a highly developed and evolved, broad body of knowledge. Many employees place themselves at a disadvantage vis-à-vis their colleagues in the absence of the right professio…
Training and Development is a highly developed and evolved, broad body of knowledge. Many employees place themselves at a disadvantage vis-à-vis their colleagues in the absence of the right professional training and development. If employees have to consistently close gaps in their learning, they need to keep…
Added by John Robinson on July 19, 2017 at 6:21am — No Comments
 How to create processes and procedures to implement them?
How to create processes and procedures to implement them?
Product Risk Management is a critical aspect of ensuring medical devices are safe and effective for intended uses. This course will help you understand the regulatory requirements, including ISO14971.
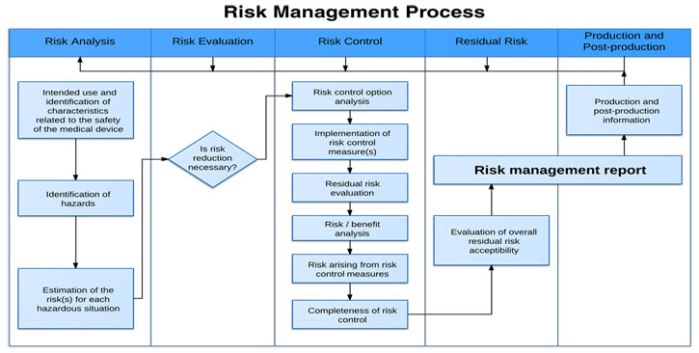
You’ll learn techniques that can help you identify hazards and potential harms. You’ll learn how to mitigate risk and effectively monitor risk to ensure your customers receive safe and effective products. A rigorous…
ContinueAdded by Adam Fleming on July 19, 2017 at 5:47am — No Comments
 Ensuring compensation-focused compliance with Stark Law considerations
Ensuring compensation-focused compliance with Stark Law considerations
Stark Law compliance is now more than just a compliance issue. It has grown to become an Enterprise Risk Management matter, if the substantial awards and settlements in recent enforcement actions are anything to go by. For healthcare organizations that develop and implement provider contracts; managing their compliance and enterprise risk by ensuring that…
ContinueAdded by Roger Steven on July 18, 2017 at 7:50am — No Comments
 Why is credit card surcharge an issue for businesses?
Why is credit card surcharge an issue for businesses?
The credit card surcharge issue has always been a tricky one in the US. Back in 2005, this issue was the subject of an antitrust lawsuit, and the resultant judgment, which came in mid-2012 prohibited credit card surcharge in ten States. Another 12 States are in the process of implementing their laws.
Although credit card regulations have traditionally opposed surcharging; companies have been circumventing merchant rules to ensure that credit card surcharge continues to be made. Even…
ContinueAdded by Adam Fleming on July 18, 2017 at 5:27am — No Comments
 Understanding GLP's and their relationship with GMPs and SOP’s
Understanding GLP's and their relationship with GMPs and SOP’s
Good Laboratory Practices (GLP’s) are a series of federal regulations passed in the US by the FDA under 21 CFR Part 58. In addition, Environmental Protection Agency (EPA) also has formed GLP’s both for Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) in 40 CFR Part 160 and for Toxic Substances Control Act (TSCA) in 40 CFR Part 792.

GLP’s are Quality…
ContinueAdded by Adam Fleming on July 17, 2017 at 5:38am — No Comments
 Entities should do everything it takes to avoid HIPAA fines and penalties
Entities should do everything it takes to avoid HIPAA fines and penalties
The fact of about 120 million individuals being affected by HIPAA breaches in 2015 highlights two important points:
- There is a mad demand for these records in the black market, which is why they are being targeted to this extent. Any wonder that PHI is in greater demand in the black market than even social security and credit…
Added by Roger Steven on July 14, 2017 at 8:05am — No Comments
 Steps to IEC 60601-1 approval
Steps to IEC 60601-1 approval
The 60601-1 is a standard that relates to the safety of medical electrical equipment. This harmonized standard is recognized by most countries around the world. Existing and new medical devices have to comply with the requirements set out in IEC 60601-1.
The IEC 60601-1 is going through its latest revision, its 3rd edition,…
ContinueAdded by Adam Fleming on July 14, 2017 at 6:27am — No Comments
 The ways of applying ISO 14971, IEC 62304 and IEC62366-1 to medical device software
The ways of applying ISO 14971, IEC 62304 and IEC62366-1 to medical device software

Diligence, complete and proper examination and assessment of the gaps, and correction of the gaps right from the very start of product development are the core characteristics that need to go into implementing risk management of software used in medical devices.
These are the reasons for it:
- Lack of proper and complete implementation and gaps in them lead to major drawbacks such as production delays or…
Added by John Robinson on July 13, 2017 at 6:47am — No Comments
Featured Blog Posts
- Nanomedicine - A Key Component to the Future of Medicine
- Medical Affairs Role in Pharmaceutical Companies
- A Workshop: What Is the Universe of a Universal Health Language?
- WINDS OF CHANGE IN COLLABORATING TO INNOVATE WITH OPEN SOURCE SOFTWARE ... Kathleen Goolsby in Open Source Delivers
- You Are Not Your Doctor’s Customer – But You Can Do Something About It
- Medical Megatrends Stem Cells – Part III
- MedTechIQ Member ... Actuated Medical ... Obtains FDA Clearance for Tubeclear System!
Latest Blog Posts
- Register ISBN Number: A Complete Guide for New Authors
- How to Get an FSSAI License for Mushroom Cultivation Business in India
- Importance of LMPC Legal Metrology Andhra Pradesh Registration in 2026
- Export Mustard Oil from India in 2025 with IOPEPC: Requirements & Benefits
- RCMC Certificate Explained: A Must-Have for Indian Exporters
- BEE Certification for LPG Stoves: Process, Benefits, and Compliance
- Everything You Need to Know About Legal Metrology Certificate Registration in Delhi
Most Popular Blog Posts
- Medical Affairs Role in Pharmaceutical Companies
- For MedTech-IQ Members ... Free GoGo Inflight Internet Service
- PricewaterhouseCoopers' Top 10 Healthcare Trends for 2010
- EDITORIAL – Conor O’Dea: A coup for Cayman Finance
- How to improve healthcare services with Visual Communication Technology
- Want to make a career in compliance in the US? - Just Upgrade Yourself $10
- Are You Obese but Healthy? You May Still Be 96% More Susceptible to Heart Failure
Monthly Archives
2026
2025
- December (11)
- November (8)
- October (3)
- September (5)
- August (6)
- July (20)
- June (19)
- May (39)
- April (34)
- March (8)
2024
2022
2020
2019
2018
- December (5)
- November (8)
- October (8)
- September (7)
- August (8)
- July (9)
- June (3)
- April (7)
- March (7)
- February (21)
- January (32)
2017
- December (11)
- November (39)
- October (64)
- September (60)
- August (34)
- July (30)
- June (22)
- May (40)
- April (16)
- March (12)
- February (18)
- January (15)
2016
2015
2014
2013
2012
- October (3)
- September (1)
- August (9)
- July (8)
- June (8)
- May (11)
- April (6)
- March (11)
- February (1)
- January (6)
2011
- December (2)
- November (3)
- October (7)
- September (3)
- July (4)
- June (4)
- April (7)
- March (4)
- February (3)
- January (7)
2010
- December (8)
- November (11)
- October (6)
- September (11)
- August (18)
- July (28)
- June (24)
- May (10)
- April (31)
- March (44)
- February (45)
- January (85)
2009
- December (104)
- November (63)
- October (44)
- September (68)
- August (53)
- July (37)
- June (50)
- May (43)
- April (63)
- March (106)
- February (36)
- January (15)
2008
1999
- November (5)
© 2026 Created by CC-Conrad Clyburn-MedForeSight.
Powered by
![]()